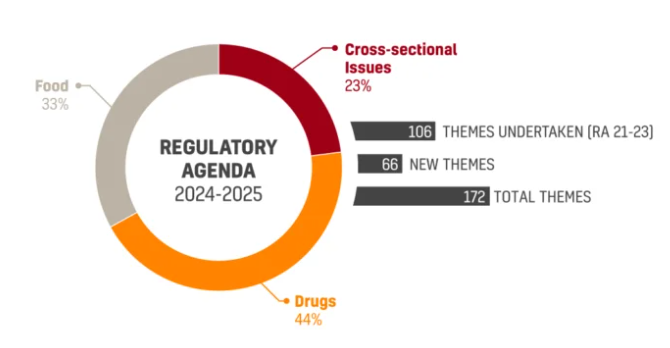
2024-2025 Regulatory Agenda Has Been Approved by the Brazilian FDA
ANVISA’s new Regulatory Agenda 2024-2025 (RA 24/25) was approved and published on the Official Gazette on December 18th. The Joint Ordinance No. 1…

Marcelo Brisolla on LinkedIn: I recommend this training to all companies that want to do business with…
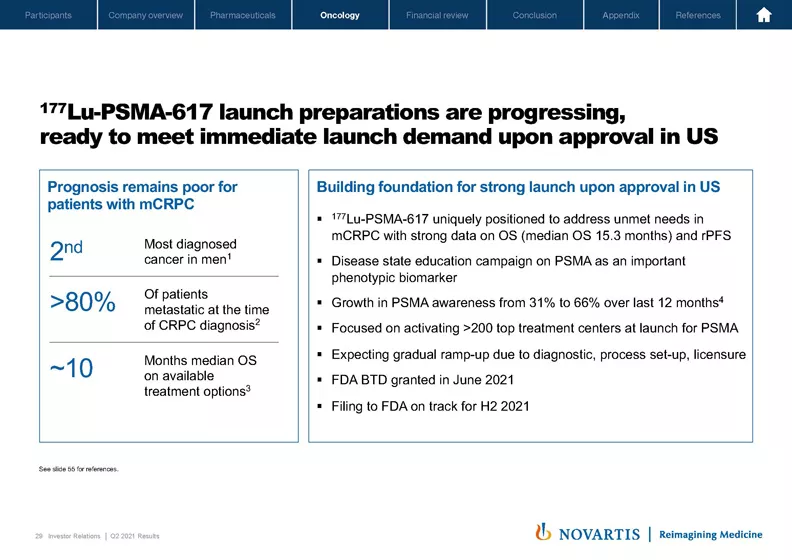
2021 Q2 Results Presentation & Transcript

FDA Updates for the Week of Feb. 5, 2024: Review Dates and Advisory Committee Meetings Scheduled

SEC Filing Alkermes plc

Jamaica: Request for an Arrangement Under the Precautionary Liquidity Line and Request for an Arrangement Under the Resilience and Sustainability Facility-Press Release; Staff Report; Staff Statement; and Statement by the Executive Director

Global Service Providers Guide 2023 by Chemical Watch - Issuu

World Sleep 2023 Scientific Program by World Sleep Society - Issuu

425

Sterile Medical Packaging Market - Manufacturers & Companies